Introduction:
I’m probably treading on thin ice here, but I’ve been thinking about why milk would cause acne in some people. I have not the means of forming a solid judgment but initially, I was thinking offhand that the high calcium content in milk could be a factor. Excess calcium impairs the absorption of zinc and a zinc deficiency depletes vitamin E and thus vitamin A. Zinc and vitamin A are particularly protective against the development of acne.1
I think this highlights how complex nutrient interactions can get as well as the importance of examining all possibilities and assumptions when we attempt to draw associations between two things. It’s tempting to assume that one thing causes another simply because they regularly occur simultaneously, or one regularly occurs before the other in time. Leaping to a cause and effect conclusion is easier and faster, no doubt, than to take due cares to investigate the relationship so as to rule out all possible alternative explanations.
Many, unfortunately, do not shoulder such care—unintentionally or not. But I leave this train of thought.
Bacteria are often said to be the cause of acne but I’ve always had my doubts about this model. Antibiotics—applied topically or taken orally—do in fact improve and prevent acne, sometimes quite dramatically, but I think the explanation as to how this happens lies outside the idea that antibiotics merely kill bacteria, P. acnes, present on the skin. Lo and behold, bacteria are not unconditionally required for acne.2
Lipid peroxidation is undoubtedly at play in the development of acne lesions. And understanding the interplay among vitamin A, vitamin E, and fatty acids I think will permit us to move away from an intolerable deal of guesswork and to a more rational approach to this seemingly implacable foe that affects people of all ages.
Vitamin A
A deficiency of vitamin A, or retinol, is not thought to be a problem among adults as much as an excess of vitamin A is. (Of course with the exception of alcoholics, since beta-carotene is metabolized to retinol by the same dehydrogenase enzyme that detoxifies alcohol to acetaldehyde. So the presence of alcohol slows the conversion of beta-carotene to retinoic acid, permitting beta-carotene to accumulate in the body.)
However, a thyroid deficiency, as well as a deficiency of retinol and vitamin B12, impairs the secretion of bile, which is needed to absorb dietary retinol.3 In other words, a retinol deficiency can reinforce itself, that is, unless the pattern can be short-circuited with a good diet.
I have to pause for a moment to say that supplementation with vitamin A should be undertaken with discretion. Reviewing the toxicological data, vitamin A interacts with many supplements and drugs, sometimes to a significant degree, and the range of doses that produce intoxication is apparently very wide: One person may be able to take hundreds of thousands of units for weeks without indications of toxicity, while another person may experience acute intoxication from just one small dose of vitamin A. There are obviously many factors at play that determine a person’s tolerance to vitamin A.
Retinol is oxidized in the skin to its more active metabolite, retinoic acid. The skin not only oxidizes retinol to retinoic acid, but it also stores retinol in the cell’s fatty regions. Under the right conditions, retinol is liberated from its storage site, and converted to retinoic acid, which can then bind to nuclear “retinoic acid receptors” to exert the full force of vitamin A’s effects. It is thus fair to suppose that vitamin A’s toxicity—which mainly results from too much retinoic acid—is buffered against by the coordinated release and oxidation of retinol.
(Could those who are more sensitive to vitamin A merely be fast metabolizers?)
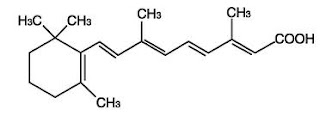
Retinol
Retinoic acid
Certain human skin cells have little capacity to esterify (i.e., store) retinol, so they must depend on the continuous uptake of retinol from the circulation. The liver has a tremendous capacity to store vitamin A: At utmost, we can say that the liver can normally store enough vitamin A to hold a person over for 5 to 10 months without any intake of vitamin A.4
(Nonetheless, it’s been known, at least since the 1920s, that although an animal that is vitamin A deficient may appear normal and healthy, yet have an impaired ability to reproduce and a tendency to breakdown in health in the prime of life compared to an animal that is not vitamin A deficient.)
Retinol—applied to the skin or taken orally—can help to fight back the excess sebum production and proliferation of skin cells initiated by lipid peroxidation processes. Retinoids are synthetic forms of vitamin A that are supposedly employed in lieu of retinol because retinol would become toxic in the doses needed to treat acne. Considering the egregious side effects associated with the use of retinoids, and despite the wide range of toxic doses associated with the use of retinol, I would still more often than not opt for retinol first.
Skin lipids
Free fatty acids and squalene are major lipids that make up sebum. Squalene is highly unsaturated in structure and highly susceptible to peroxidation and photodegradation. The byproducts, squalene peroxides, promote acne, roughening of skin, and wrinkling.5,6 The free fatty acids, when polyunsaturated, degenerate to promote the peroxidation of nearby lipids, including squalene, whereas saturated fats do not.
Squalene
Oils applied to the skin are readily metabolized, and investigations in which oils are applied to skin cells have provided insights into the role of different lipids in the pathophysiology of skin-related disorders—including acne. Applying unsaturated fatty acids to the skin, for instance, almost instantly causes skin cells to take up calcium.7 This influx of calcium leads to abnormal keratinization in follicles and, subsequently, the plugging of pores, encouraging acne development.
Intracellular calcium also liberates PUFA, activates lipoxygenases (LOX), which convert PUFA to lipid hydroperoxides that are then rapidly reduced to lipid hydroxides, and, by activating perixosome-proliferater activated receptors (PPAR) in the skin, increase the expression of cyclooxygenases (COX), which convert PUFA to prostaglandins. These PUFA oxidation products further reinforce the conditions that favor acne development. COX inhibitors, applied topically, are routinely used to treat acne, and a LOX inhibitor, zileuton, has been shown to be effective in treating acne as well—topically and orally.8
Because of their bulky conformation, unsaturated fatty acids also reduce the skin’s barrier integrity, which leads to abnormal keratinization and clogging of pores, too.9 Saturated and trans fats are weak PPAR activators, not substrates for LOX or COX, and very long chain saturated fatty acids (with cholesterol) enhance the skin’s barrier function. Medium chain saturated fats, such as lauric acid, can worsen acne in some people by activating toll like receptors (TLR) in the skin. Sebum from pre-pubertal children contain more omega-9 fatty acids and less omega-6 fatty acids compared to sebum from adolescents when acne begins to appear.
Vitamin E
A vitamin E deficiency, or an excess of polyunsaturated fat, depletes vitamin A. This is because while vitamin E, an antioxidant, spares vitamin A from being degraded, it also prevents the oxidation of polyunsaturated fats. So when polyunsaturated fats are present in tissues in large amounts, less vitamin E is available to protect vitamin A from degradation. Generally, our requirement for vitamin E increases as our consumption of polyunsaturated fat increases.10
It has been calculated that at least 0.6 milligrams of vitamin E are needed for every gram of polyunsaturated fat ingested. But the protection provided by vitamin E diminishes as increasing amounts of polyunsaturated fats are ingested.11
Although toxicity to large amounts of vitamin E has not been definitively shown before now, large amounts of vitamin E can be protective in some contexts. For instance, in areas of heavy air pollution, in which levels of ozone and other atmospheric oxidants are high, large amounts of vitamin E can help to protect the lungs from tissue damage.12 Further, large amounts of vitamin E help to promote repair of tissue following burn injuries.13
Such protection is apparently direct and indirect. That is, vitamin E not only mops up free oxygen, but by sparing vitamin A, known as the “anti-infection vitamin,” vitamin E prevents damage to the epithelial cells (i.e., skin) of the body. Vitamin A, in turn, enhances the efficacy of vitamin E in giving resistance to diseases. And good thyroid function increases our requirement for both vitamin A and vitamin E.
Vitamin E is also known as the “anti-sterility” vitamin, and I think this is in part due to its ability to spare vitamin A, which protect skin surfaces, including the skin that lines the gonads.
Vitamin E, over the years, has had lavish claims made on its behalf as to preventing and curing various conditions; after reviewing the bare facts, however, not much more can be said about it at this point.
Conclusion
The development of acne is beginning to be seen as having an inflammatory origin, in which lipid peroxidation processes take center stage. I’ve discussed this elsewhere in a different context, but the eicosanoid metabolites derived from lipoxygenase enzymes—namely 15-HETE and LTB4—as well as their parent compound, arachidonic acid, are ligands for PPAR activation in the skin’s sebaceous glands.14 PPAR activation, in turn, increases the expression of COX, which generates prostaglandin E2 in in the skin to cause abnormal skin cell proliferation and lipogenesis therein, as well as inflammation.
Danny Roddy has written an interesting blog post about the role of prostaglandin E2 in hair loss. We can now, tentatively, add acne to the list of conditions impacted negatively by COX and prostaglandin E2.
Recall that the presence mead acid, generated when the diet is deficient in the essential fatty acids, inhibits the generation of LTB4, and salicylates (and generally NSAIDs) inhibit the generation of prostaglandin E2. Vitamin E is carried to and from the skin’s surface by way of sebum, so topically applied vitamin E could help to put a brake on the lipid peroxidation processes that lead to the development of full-blown acne. Selenium works with vitamin E to suppress random oxidation processes in the skin. Schisandra fruit (dried or fresh), tea, and cannabis (leaf) contain natural LOX inhibitors and are generally safe to use.15
References
1. Brandt, S. The clinical effects of zinc as a topical or oral agent on the clinical response and pathophysiologic mechanisms of acne: a systematic review of the literature. Journal of drugs in dermatology : JDD 12, 542–5 (2013).
2. Zouboulis, C. C. et al. What is the pathogenesis of acne? Experimental dermatology 14, 143–52 (2005).
3. Mandal, S. K. & Dastidar, A. G. Hypothyroidism as a possible aetiology of vitamin A deficiency. Journal of the Indian Medical Association 83, 339–40 (1985).
4. Guyton, A. & Hall, J. Textbook of Medical Physiology. 1104 (Saunders: 2006).
5. Chiba, K., Yoshizawa, K., Makino, I., Kawakami, K. & Onoue, M. Comedogenicity of squalene monohydroperoxide in the skin after topical application. The Journal of toxicological sciences 25, 77–83 (2000).
6. Chiba, K., Sone, T., Kawakami, K. & Onoue, M. Skin roughness and wrinkle formation induced by repeated application of squalene-monohydroperoxide to the hairless mouse. Experimental dermatology 8, 471–9 (1999).
7. Katsuta, Y., Iida, T., Inomata, S. & Denda, M. Unsaturated fatty acids induce calcium influx into keratinocytes and cause abnormal differentiation of epidermis. The Journal of investigative dermatology 124, 1008–13 (2005).
8. Zouboulis, C. C. et al. A new concept for acne therapy: a pilot study with zileuton, an oral 5-lipoxygenase inhibitor. Archives of dermatology 139, 668–70 (2003).
9. Yamamoto, A., Takenouchi, K. & Ito, M. Impaired water barrier function in acne vulgaris. Archives of dermatological research 287, 214–8 (1995).
10. Meydani, M. Vitamin E requirement in relation to dietary fish oil and oxidative stress in elderly. EXS 62, 411–8 (1992).
11. Valk, E. E. & Hornstra, G. Relationship between vitamin E requirement and polyunsaturated fatty acid intake in man: a review. International journal for vitamin and nutrition research. Internationale Zeitschrift für Vitamin- und Ernährungsforschung. Journal international de vitaminologie et de nutrition 70, 31–42 (2000).
12. Elsayed, N. M., Mustafa, M. G. & Mead, J. F. Increased vitamin E content in the lung after ozone exposure: a possible mobilization in response to oxidative stress. Archives of biochemistry and biophysics 282, 263–9 (1990).
13. Kuroiwa, K. et al. Metabolic and immune effect of vitamin E supplementation after burn. JPEN. Journal of parenteral and enteral nutrition 15, 22–6
14. Thuillier, P. et al. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. The Biochemical journal 366, 901–10 (2002).
15. Schneider, I. & Bucar, F. Lipoxygenase inhibitors from natural plant sources. Part 1: Medicinal plants with inhibitory activity on arachidonate 5-lipoxygenase and 5-lipoxygenase[sol ]cyclooxygenase. Phytotherapy research : PTR 19, 81–102 (2005).