Purely based on physical and chemical considerations, it’s becoming clear that higher membrane saturation indices go hand in hand with longevity and a greater resistance to toxic substances like lipopolysaccharide.
The types of saturated fats and unsaturated fats, as well as the proportion of each in the cellular membranes, are governed largely by the types of fats ingested and the body’s nutritional status and hormonal milieu. It’s been touched on previously on this blog but insulin, for instance, activates several desaturase enzymes, which explains why fats synthesized from carbohydrates, via DNL, consist of a mixture of saturated & monounsaturated fats. (On the other hand, food restriction tends to increase the saturation indices of membranes, and not simply because food-restricted individuals are incidentally ingesting less unsaturated fats, as I’ve recently heard suggested off hand.)
These inherent processes, whereby saturated fats are converted to unsaturated fats, seem to be important for controlling the fluidity of cellular membranes so that enzymes, such as lipases, which only operate within a range of membrane viscosities, can act on cellular lipids. Oxygen and the reducing equivalents, NADH & NADPH, are essential cofactors of the desaturase enzymes. (Fructose depletes NADH.)
Thyroid supplement users should take note of the fact that thyroid hormone tends to decrease the saturation indices of membranes.
Thyroid hormone, in parallel with insulin, decreases the saturation indices of membranes by activating certain desaturase enzymes (Δ5- and Δ6-desaturases), thereby increasing the proportion of the highly unsaturated fats, like arachidonate, to their parents, like linoleate (Van Doormaal, Muskiet, Martini, & Doorenbos, 1986).
(Simultaneously, the proportion of saturated fats to unsaturated fats in the blood also decreases, and there is a mass transfer of cholesterol from the blood and into the tissues upon the exposure to thyroid hormone, T3, whereby blood cholesterol levels decrease and the stability of cellular membranes remains, more or less, unchanged [Ruggiero, Landriscina, Gnoni, & Quagliariello, 1984].)
These subtle changes in the composition of cellular membranes have not-so-subtle implications on the chemical interactions & physical ordering of the cell itself. Unsaturated fats have at least one double bond, and so the carbon atoms that are joined by the double bond(s) share 4 electrons, rather than 2, resulting in a greater density of negative charges, and thus a greater so-called “inductive effect” on surrounding molecules. The double bonds also impart a slight bend to unsaturated fats, and this, among other things, alters the functioning of the proteins that operate in the vicinity and allows for certain unsaturated fats to be metabolized by enzymes that yield the eicosanoids (e.g., prostaglandins).
Interestingly, cancer cells are characterized by (1) lower membrane saturation indices, (2) increased proportion of cholesterol to phospholipids, (3) decreased expression of the monooxygenase enzymes that propagate lipid peroxidation processes, and (4) increased expression of the enzymes that neutralize reactive aldehyde fragments (Galeotti, Borrello, Minotti, & Masotti, 1986). These are probably adaptive mechanisms in response to high rates of energy generation via glycolysis (characteristic of cancer cells), which as a consequence produces reactive fragments that can glycate labile amino groups found in proteins and lipids, at a high rate.
The rate at which energy can be generated in mitochondria is limited by the fluidity of their membranes; that is, a higher rate of energy generation (ATP) is possible when the membrane saturation index is higher than when it’s lower (Gabbita, Butterfield, Hensley, Shaw, & Carney, 1997). It’s conceivable that the lipid composition of the inner mitochondrial membrane in which the electron accepting protein complexes reside, directly impacts the efficiency with which ADP is phosphorylated to ATP (e.g., calorie restriction, again, increases the saturation indices of membranes and this in turn, increases—not decreases—the rate of respiration & generates excessive amounts of ATP [Lin et al., 2002]).
I’ve discussed this elsewhere, but ATP—not the membrane lipid bilayer, which likely assumes a passive role—is the basis for the structural ordering and the interactions among cellular water, proteins, ions, and other small molecules, in what Dr. Gilbert Ling calls the cell’s “resting-living state.” In other words, ATP is synonymous with life; it’s the basis for how we fundamentally perceive, adapt, anticipate, regenerate, and cope with ever-present changes (i.e., stress) in the environment—not for powering a membrane pump that supposedly maintains a gradient of ions across the plasma membrane.
Considering the fact that small changes in the structural elements in cells lead to drastic changes in their biochemical functioning, and the fact that molecules in cells are in constant motion, in the context of cells oscillating back and forth between the energetically favored “resting-living state” and the transiently destabilized “active-living state,” the ability of cells to hold themselves together, so to speak, and to maintain order and coherence in the face of an ever-changing environment is truly elegant and remarkable.
Given the discussion above, I think people who are interested in increasing their thyroid hormone levels—via drugs or diet—should be especially careful about avoiding the excessive consumption of the “essential fatty acids.” Saturated fats—synthesized from carbohydrates or directly ingested—will flow down the ω-9 fatty acid biosynthetic pathway, leading to the production of monounsaturated fats and polyunsaturated fats with 3 or 4 double bonds, rather than 5 or 6, as is the case with ω-3 and ω-6 fats(figure 1).
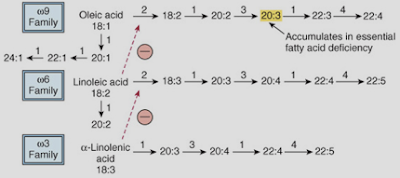 |
| Figure 1: fatty acid biosynthetic pathways |
Cholesterol also has a modulating effect on the activity of the desaturase enzymes, decreasing the synthesis of the highly unsaturated fatty acids (Brenner, Bernasconi, González, & Rimoldi, 2002; Garg, Sebokova, Thomson, & Clandinin, 1988). Sucrose, namely its fructose half, increases the production of cholesterol more than any other single nutrient.
References
Brenner, R. R., Bernasconi, A. M., González, M. S., & Rimoldi, O. J. (2002). Dietary cholesterol modulates delta6 and delta9 desaturase mRNAs and enzymatic activity in rats fed a low-eFA diet. Lipids, 37(4), 375–83. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12030318
Gabbita, S. P., Butterfield, D. A., Hensley, K., Shaw, W., & Carney, J. M. (1997). Aging and caloric restriction affect mitochondrial respiration and lipid membrane status: an electron paramagnetic resonance investigation. Free radical biology & medicine, 23(2), 191–201. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9199881
Galeotti, T., Borrello, S., Minotti, G., & Masotti, L. (1986). Membrane alterations in cancer cells: the role of oxy radicals. Annals of the New York Academy of Sciences, 488, 468–80. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3555261
Garg, M. L., Sebokova, E., Thomson, A. B., & Clandinin, M. T. (1988). Delta 6-desaturase activity in liver microsomes of rats fed diets enriched with cholesterol and/or omega 3 fatty acids. The Biochemical journal, 249(2), 351–6. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=1148710&tool=pmcentrez&rendertype=abstract
Lin, S.-J., Kaeberlein, M., Andalis, A. A., Sturtz, L. A., Defossez, P.-A., Culotta, V. C., Fink, G. R., et al. (2002). Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature, 418(6895), 344–8. doi:10.1038/nature00829
Ruggiero, F. M., Landriscina, C., Gnoni, G. V, & Quagliariello, E. (1984). Alteration of plasma and erythrocyte membrane lipid components in hyperthyroid rats. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et métabolisme, 16(1), 37–40. doi:10.1055/s-2007-1014688
Van Doormaal, J. J., Muskiet, F. A., Martini, I. A., & Doorenbos, H. (1986). Changes in fatty acid profiles of plasma, erythrocytes and polymorphonuclear leukocytes in induced hypothyroidism in man: indirect evidence for altered delta 6 desaturase activity. Clinica chimica acta; international journal of clinical chemistry, 156(3), 299–313. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3521951