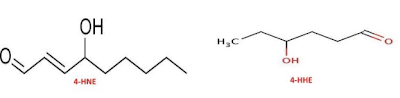
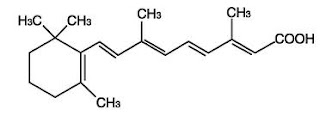
Before getting into this post, I have one piece of news to announce. I’ll be writing for Matt Stone’s January newsletter, probably on the topic of physical attractiveness. You can sign up for the newsletter now before the inaugural December issue is sent out.
This topic, which I’ve written a blog post about[*] with regard to the impact of stress on facial symmetry, I think is important given the warped view people apparently have of their bodies, and it is crystal clear to me now that a big part of the problem is, I hate to say it, probably the most popular diet movement now: the paleo/primal movement.
This issue is closer to me than I care to say. The pursuit of beauty in Korean culture has reached a point of sickness, even among guys, to where more people at younger ages are opting for, or at least thinking about, plastic surgery, procedures that are not necessarily free of invasiveness. I don’t understand their ideals as to what constitutes beauty but, like the situation here, they are, in effect, manufacturing the traits that the opposite sex finds attractive to give off the perception of health, rather than doing the opposite: manufacturing health to promote physical attractiveness. And there is quite a bit of evidence in the literature, which I’ve been reading casually again of late, to suggest that the latter approach is, within reason, legitimate. That is to say, physical attractiveness is reflection of a person’s genetic, reproductive, emotional, mental, and physical health, all of which are amenable to the appropriate environmental factors.
This topic, which I’ve written a blog post about[*] with regard to the impact of stress on facial symmetry, I think is important given the warped view people apparently have of their bodies, and it is crystal clear to me now that a big part of the problem is, I hate to say it, probably the most popular diet movement now: the paleo/primal movement.
This issue is closer to me than I care to say. The pursuit of beauty in Korean culture has reached a point of sickness, even among guys, to where more people at younger ages are opting for, or at least thinking about, plastic surgery, procedures that are not necessarily free of invasiveness. I don’t understand their ideals as to what constitutes beauty but, like the situation here, they are, in effect, manufacturing the traits that the opposite sex finds attractive to give off the perception of health, rather than doing the opposite: manufacturing health to promote physical attractiveness. And there is quite a bit of evidence in the literature, which I’ve been reading casually again of late, to suggest that the latter approach is, within reason, legitimate. That is to say, physical attractiveness is reflection of a person’s genetic, reproductive, emotional, mental, and physical health, all of which are amenable to the appropriate environmental factors.
Unfortunately, with the improvements in the advancements of surgical procedures and the sophistication with which makeup is applied, manufacturing physical attractiveness is not hard to do. I can’t help but think, if only they would put as much effort into curing a disease . . . I kid. Well, sort of.
For regular readers, it goes without saying that stress is a major theme of this blog. And given the centrality of the stress to all things concerned with health, metabolism, aging, and disease, as well as its fundamental importance to understanding those concepts, I’ve chosen to do this by way of rational deliberation. So you can bet that I will probably incorporate stress, as it relates to physical attractiveness, in the newsletter. But as for the rest of this post, I’ll provide some background information – in particular on stress – on the things I’ll probably discuss in that newsletter article, as a means to satisfy my urge right now to put fingers to keyboard.
I think most of us know, or are at least aware of, the relationship between stresses and how we look, feel, and perform in life. However, you may not know that that intuition is grounded in a relatively big body of scientific evidence.
One hormone, the main focus of attention in the literature in this regard although there are others, is cortisol.[†] The regulation of cortisol is surprisingly complex, and to elucidate the mechanisms by which it contributes to diseases has been a challenge. Although there still exists a gap, which may not be filled anytime soon (owing partly to not even having an idea of how to do so), some have merely closed that gap in light of the side effects inextricably linked to Cushing’s syndrome, a condition where cortisol is secreted excessively. The idea is that because people with the syndrome develop cardiovascular disease, diabetes, fat in morbid places, thinning skin, etc., healthy people (or those who are just below the point of clinical detection for Cushing’s) who are afflicted by the same conditions are overexposed to cortisol, too. A leap of faith unjustified without the requisite data to do so, to say the least.
What is clear is that the regulation of cortisol goes off the rails and out of control as we age and in disease. I won’t go into why this happens here, but the effect is that our exposure to cortisol has a tendency to increase, whereas in youth and in health, upon the exposure to a stressor, cortisol should come in, do its thing, and shortly (this is the operative word) thereafter be cleared from the body. In fact, all the stress hormones should work this way. But in old age and in disease, cortisol is not only secreted in excess upon the exposure to the same stressor, but it also persists in the blood longer; these are the conditions whereby irreversible (another operative word) degenerative changes begin to develop that, in an insidious fashion, weakens our ability to bounce back from subsequent stressors.[‡]
I think it’s probably worth pausing here to clearly define what stress is. Fundamentally, it entails a mismatch; a mismatch between the stressors we are exposed to and our ability to mount an effective response so as to restore the balance[§]that was disturbed by that stressor. That’s it. So it’s clear that a stressor can be anything; in this light, an immune reaction to getting a flu shot or the characteristic changes that occur in adipose tissue from becoming fatter are considered stressors.
Okay, let’s get back on track. . . .
If you’ve seen what young guys in Korea look like, or I should say the look they strive for, it’s funny and sad at the same time. They look like pretty women, straight up. I should say that all the traits that they strive for are the traits that, from a rudimentary perspective, are the ones that are at odds with what the opposite sex finds attractive.[**] Paunch fat,[††] for instance, is a sign of dominance and power in men, a feature that has probably been sexually selected for. In fact, having some extra fat – not being rail thin or having the bodily dimensions of a woman – may not only be inherently healthier, but may also serve as cues for a robust immune system and reproductive health, which is probably why this is feature that women have historically found attractive, more so than the secondary sex characteristics, for instance. Just saying.
(Correspondingly, big boobs, slightly protruding paunches, and curvy thighs and butts are some of the forms and shapes men inherently find attractive; it’s only now that the anorexic look has assumed the role of the ideal body form among women.)
Excessive stress, in the most basic terms, leads to excessive exposure to cortisol (which is probably why, whatever this is worth, low-carbohydrate dieters start to take on the Cushing’s face). Cortisol shares an inverse relationship with testosterone, and this is the pattern – low testosterone and high cortisol – that has been distinctly found to associate with the traits (e.g. even sweat smell) that deters the opposite sex. That’s not to say that high testosterone levels, even in the high normal physiological range, is ideal, either. You could read those studies rather than accept my position here; in the midst of doing that, you’ll see how complex it can get, especially after adding psychological factors, early life experiences, and developmental variables into the mix. Suffice it to say here, higher is not necessarily better, and does not correlate well at all with perceived attractiveness; higher levels also correlate with poorer performances on certain cognitive abilities.
Chronic and excessive stress, in the most broadest sense, not only kills, but it also ruins our health and therefore impacts negatively on our physical attractiveness. The ability to bounce back (you get my meaning now I hope), is therefore an indicator of how much stress we’ve sustained, and how effectively we’ve dealt with that stress. Manufacturing physical attractiveness to incidentally give off the perception of health (e.g. reproductive health) is as backwards as it could get. So is conforming your body shape to that which is deemed merely culturally acceptable, at the expense of your physical, psychological, and emotional wellbeing.
It is important to bear in mind, if you haven’t picked up on it already, that the experiments in which the traits considered attractive or unattractive by the opposite sex were compared with measures, such as salivary hormone concentrations, are correlative in nature; the difficulty lies in converting that correlative data into proof of cause and effect; unfortunately, I don’t think we have the slightest idea of the means to do that.
Be that as it may, an important point is that men and women know, and generally agree upon, what they find attractive in the opposite sex, and that the concept of physical attractiveness is not so much in the eye of the beholder, as much as it is seated in biology, and therefore amenable to environmental influences such as stress. Beauty is also not merely skin deep; it is, I believe, a real indicator of our overall health. Incidentally, it serves to explain why physical attractiveness correlates fairly well with social status, financial status, and other traits such as social competence, intelligence, and affability.
I hate to leave readers hanging, but the reason for writing this post was to generate thought on the topic, as well as anticipation for the newsletter article I mentioned at the outset. In that article, I plan to discuss some of the means to help to regulate the stress hormones, and to prevent the apparently inevitable decline in the tissues’ responsiveness to those hormones.
I also plan to discuss the possible role of a certain neurotransmitter in physical attractiveness. Neurotransmitters, from my own observations, are egregiously misunderstood and oversimplified to the point where their discussion becomes meaningless. Deliberately or not, people have blurred the line between the function and metabolic effects of certain neurotransmitters in the central nervous system and in the body. A case in point is serotonin. And that sloppy belief has become pervasive because, well, that’s just how the Internet works.
Anyway, look forward to that. I hate to be vague but I’ll be busy for a while so I can’t say when I’ll post again here. Regardless, I want to take this occasion to thank all the readers for, well, reading. I also want to thank the people who have sent me messages and emails of support; I’ve had some really interesting exchanges through these communications, especially with the people who are as open-minded as I strive to be. I hope, in my short stint, I have provided content worth your while; a respite from the blogs that require a Ph.D. to demystify (and seem to be written for no other purpose than to stroke the writer’s own ego, without regard for the audience) and the drove of health and nutrition blogs devoted to regurgitate, over and over again, a certain way of eating and living.[‡‡]
[*] An early blog post that was taken down due to overwhelming formatting issues that I didn’t want to deal with for one more second of life.
[†] If you recall, cortisol was the main hormone of interest of Han Selye.
[‡] Selye said that this ability to bounce back was limited by a predetermined amount of “adaptational energy” with which we were genetically endowed. Suffice it to say here, he was probably incorrect in his belief.
[§]“Homeostasis,” technically.
[**] Unless of course the goal is to look like a pretty woman in which case, so be it. Even if I try, I will never fully understand Korean culture.
[††] Fat located on the outer ventral wall of the abdomen, centered on the belly button.
[‡‡] Because I wanted to get a blog post out before the end of the weekend so that I could attempt to be productive on Monday, I wrote this one quite fast today. So it probably suffered in quality, seeing as how I didn’t have a chance to edit it in the way I would’ve liked. Please bear with me on any writing and/or grammatical errors you may find.










.gif)
.png)